Refine by
Coronary Bypass Graft Equipment & Supplies
15 equipment items found
Manufactured by:Voss Medical Products based inSan Antonio, TEXAS (USA)
HE VM/CV Coronary By-Pass Marker: Easier to use. Easier to locate and remove. The VM/CV Coronary By-Pass Graft Marker provides a blue, radiopaque marker that is easier to locate, cut, bend and remove and is suitable for patients requiring ...
by:XELTIS BV based inEindhoven, NETHERLANDS
The XABG device is a restorative, synthetic, electrospun blood vessels for CABG surgery, overtime turning into a living vessel made of patient’s own ...
Manufactured by:Celixir plc based inStratford-upon-Avon, UNITED KINGDOM
PHASE 2 : Our first investigational cardiac regenerative medicine consists of iMP cells. iMP cells are designed to treat patients with ischaemic heart disease – ischaemic cardiomyopathy – undergoing coronary artery bypass graft (CABG). They are injected around cardiac scar tissue via a single application during bypass ...
Manufactured by:XyloCor Therapeutics based inWayne, PENNSYLVANIA (USA)
XC001 is an investigational gene therapy for the treatment of patients with refractory angina with no treatment options. Patients who have exhausted pharmacologic options and are not eligible for PCI or coronary artery bypass grafts become sedentary because of their symptoms and this can exacerbate comorbidities causing rapid deterioration of ...
Manufactured by:AtriCure, Inc. based inMason, OHIO (USA)
The Isolator Synergy ablation clamp is the FIRST and ONLY surgical ablation device offered with FDA approval for the treatment of Atrial Fibrillation (Afib) and the only device either Catheter or Surgical to be approved for the treatment of persistent and long-standing persistent ...
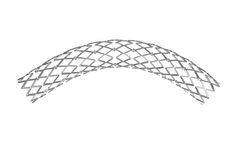
Manufactured by:amg International GmbH based inWinsen, GERMANY
The ARTHOSPico is a multicellular Cobalt-Chromium stent that offers strength, stability, and flexibility for treating the most difficult lesions. The Co-Cr Stent is indicated for patients with coronary heart disease. It is inserted in coronary vessels with a diameter of 2.0 to 4.0 mm. It is used for the treatment of de-novo and restenotic lesions in ...
Manufactured by:Bentley InnoMed GmbH based inHechingen, GERMANY
This extraordinary product combines a highly flexible one-layer stent graft with a low-profile balloon catheter to offer an unrivalled small guiding catheter compatibility. The cobalt-chrome stent platform is covered with a micro-porous ePTFE membrane. The BeGraft Coronary Stent System is not for available in the ...
Manufactured by:XyloCor Therapeutics based inWayne, PENNSYLVANIA (USA)
Coronary Artery Bypass Graft Surgery or CABG is a procedure used to treat coronary artery disease – the narrowing or blockage of the blood vessels that supply oxygen and nutrients to the heart muscle. During CABG, a healthy artery or vein from the body is connected, or grafted, to the blocked ...
Manufactured by:Baymed Inovative Health Products Inc. based inMerkez/Kilis, TURKEY
Our range includes single drapes and sets developed for cardiothoracic surgery, including heart valve replacements, pacemaker surgery, thoracotomy and coronary artery bypass ...
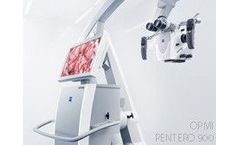
Manufactured by:Carl Zeiss Meditec AG based inJena, GERMANY
OPMI PENTERO 900 represents the next generation visualization system. Building on the innovations of OPMI Pentero introduced in 2004, OPMI PENTERO 900 combines award-winning design concepts and new functionalities in a proven, fully integrated platform. Key functions have been enhanced and new visualization methods integrated, raising OPMI PENTERO 900 to a high level of ...
by:tiakis BIOTECH AG based inKiel, GERMANY
Tiprelestat is a pioneering anti-inflammatory and tissue protective drug being developed for the treatment of pulmonary-arterial hypertension, the prevention of serious disease progression of COVID-19 and the prevention of postoperative inflammatory ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Aprotinin is a bovine pancreatic trypsin inhibitor (BPTI) inhibitor which inhibits trypsin and chymotrypsin with Kis of 0.06 pM and 9 nM, ...
Manufactured by:Palisade Bio based inCarlsbad, CALIFORNIA (USA)
Our lead asset LB1148 is a novel oral liquid formulation of the well-characterized digestive enzyme inhibitor, tranexamic acid (“TXA”), with potential to both reduce abdominal adhesions and help restore bowel function following surgery. The therapy is being developed for administration prior to major surgeries that are at risk of disrupting the intestinal epithelial ...
Manufactured by:Vascudyne, Inc. based inStillwater, MINNESOTA (USA)
Synthetic vascular grafts currently used in clinic have poor long term outcomes and low patency rates. Hyperplasia, calcification, foreign body immune response, infection, and atheroma formation are commonly encountered limiting the use of synthetic grafts, with none available for small diameter applications like coronary ...
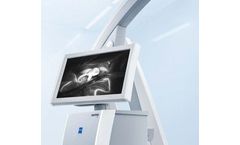
Manufactured by:Carl Zeiss Meditec AG based inJena, GERMANY
INFRARED 800 from ZEISS enables intraoperative visual assessment of blood flow and vessel patency during arteriovenous malformation (AVM), bypass, aneurysm and anastomosis surgery. ZEISS INFRARED 800 is indicated for use in neurosurgery, plastic and reconstructive procedures and coronary artery bypass graft ...